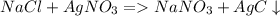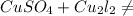Balance the following chemical reactions:
1. Pb(NO₃)₂ → PbO + NO₂ + O₂
2. CH₄...

Balance the following chemical reactions:
1. Pb(NO₃)₂ → PbO + NO₂ + O₂
2. CH₄ + O₂ → CO₂ + H₂O
3. Cu + AgNO₃ → Cu(NO₃)₂ + Ag
4. MnO₂ + HCl → MnCl₂ + H₂O + Cl₂
5. Pb(NO₃)₂ + NaCl → PbCl₂ + NaNO₃
Write the chemical reaction and balance it.
1. A solution of potassium chloride when mixed with silver nitrate solution, an insoluble
white substance is formed.
2. Copper sulphate on treatment with potassium iodide precipitates cuprous iodide(Cu₂l₂),
liberates iodine gas and also forms potassium sulphate.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Questions

Biology, 29.07.2019 04:30

Mathematics, 29.07.2019 04:30


Mathematics, 29.07.2019 04:30




Advanced Placement (AP), 29.07.2019 04:30

English, 29.07.2019 04:30



History, 29.07.2019 04:30

Mathematics, 29.07.2019 04:30




English, 29.07.2019 04:30