
Chemistry, 16.04.2020 20:01 Jerryholloway5871
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
2NaN3(s)-> 2Na(s) + 3N2(s)
An unknown amount of sodium azide (NaN3) reacts and produces 744 mL of nitrogen gas. What mass of sodium metal is produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
...
...
Questions

Biology, 09.03.2021 14:00


Mathematics, 09.03.2021 14:00




History, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00


Social Studies, 09.03.2021 14:00

English, 09.03.2021 14:00

English, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00



Geography, 09.03.2021 14:00

Biology, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00



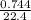 = 0.03moles
= 0.03moles 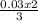 = 0.02mole
= 0.02mole

