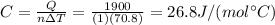A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature of 25.0°C. 118.7 g of tin metal at a temperature of 100°C is added. The final temperature in the calorimeter is 29.2°C. What is the molar heat capacity of the tin? The molar heat capacity of water is 75.4 J / (mol•°C). Assume that the heat capacity of the coffee cup is negligible.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature...
Questions

English, 10.09.2020 01:01


Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01






Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01



Mathematics, 10.09.2020 01:01



Mathematics, 10.09.2020 01:01



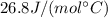
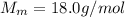
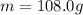
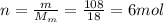
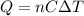
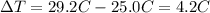 is the change in temperature of the water
is the change in temperature of the water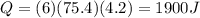
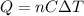
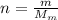 is the number of moles of tin, where
is the number of moles of tin, where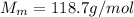 is the molar mass of tin
is the molar mass of tin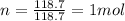
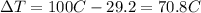 is the change in temperature of the tin
is the change in temperature of the tin