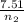A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
th...

Chemistry, 16.04.2020 23:03 Arianaagosto
A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
then added to the container until it reaches a final volume of 13.9 L. Assuming the
pressure and the temperature of the gas remained constant, calculate the moles of gas
added to the container.
15.1 mol
O 5.5 mol
0 4.40 mol
12.8 mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Questions

Mathematics, 24.12.2019 22:31





Health, 24.12.2019 22:31














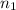 ) = 7.51 moles
) = 7.51 moles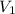 ) = 8.15 L
) = 8.15 L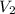 ) = 13.9 L
) = 13.9 L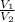 =
= 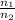
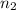 represents the final moles
represents the final moles 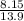 =
=