
Chemistry, 02.10.2019 12:00 janahiac09
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m o2. at this temperature, kc equals 1.4 × 102 for the reaction: 2 co(g) + o2(g)⇌2 co2(g). what is the equilibrium concentration of co?
a) 7.1 × 10-7 m.
b) 8.4 × 10-4 m.
c) 1.4 × 10-2 m.
d) 1.2 × 10-1 m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m...
Questions



Mathematics, 02.09.2021 20:30






History, 02.09.2021 20:40

English, 02.09.2021 20:40

Mathematics, 02.09.2021 20:40

Arts, 02.09.2021 20:40

English, 02.09.2021 20:40

Arts, 02.09.2021 20:40

Mathematics, 02.09.2021 20:40




Social Studies, 02.09.2021 20:40

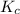
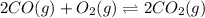
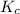 for above reaction is:
for above reaction is:![K_c=\frac{[CO_2]^2}{[CO]^2[O_2]}](/tpl/images/0282/9569/3f046.png)
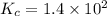
![[CO_2]=0.0010M](/tpl/images/0282/9569/bba46.png)
![[O_2]=0.0100M](/tpl/images/0282/9569/e6846.png)
![1.4\times 10^2=\frac{(0.0010)^2}{[CO]^2\times (0.0100)}](/tpl/images/0282/9569/4f227.png)
![[CO]=\sqrt{\frac{(0.0010)^2}{0.0100\times 1.4\times 10^2}}](/tpl/images/0282/9569/54368.png)
![[CO]=8.4\times 10^{-4}M](/tpl/images/0282/9569/712c0.png)


