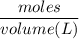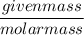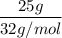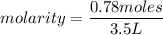What is the molarity of a methanol solution that contains 25 g of methanol in 3.5 L of a
solut...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Questions


History, 24.08.2019 16:20

Mathematics, 24.08.2019 16:20

English, 24.08.2019 16:20

Physics, 24.08.2019 16:20

Social Studies, 24.08.2019 16:20



Mathematics, 24.08.2019 16:20


Social Studies, 24.08.2019 16:20




Biology, 24.08.2019 16:20


English, 24.08.2019 16:20

Social Studies, 24.08.2019 16:20


Social Studies, 24.08.2019 16:20