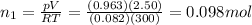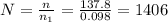Chemistry, 17.04.2020 22:16 gizmo50245
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is used to fill balloons to a volume of 2.50 L at 732 mm Hg and 27 ∞C. How many balloons can be filled with He? Assume that the cylinder can provide He until its internal pressure reaches 1.00 atm (i. e., there are 131 atmospheres of usable He in the cylinder).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3


Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

You know the right answer?
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is...
Questions

Spanish, 26.08.2019 11:30

Geography, 26.08.2019 11:30

Health, 26.08.2019 11:30


Physics, 26.08.2019 11:30



Chemistry, 26.08.2019 11:30

Biology, 26.08.2019 11:30

Social Studies, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

History, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

Geography, 26.08.2019 11:30

Mathematics, 26.08.2019 11:30

English, 26.08.2019 11:30


Mathematics, 26.08.2019 11:30

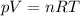
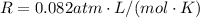 is the gas constant
is the gas constant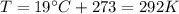 is the temperature
is the temperature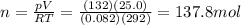
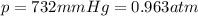 is the pressure
is the pressure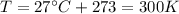 is the temperature
is the temperature