-50100 J

Chemistry, 18.04.2020 01:04 rivera6681
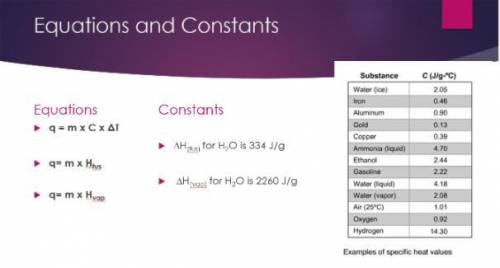
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-339,000 J
-627 J
-307.5 J

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-50100 J
Questions

Mathematics, 22.08.2019 23:40

Mathematics, 22.08.2019 23:40

Mathematics, 22.08.2019 23:40

History, 22.08.2019 23:40

History, 22.08.2019 23:40


Mathematics, 22.08.2019 23:40

English, 22.08.2019 23:40


Mathematics, 22.08.2019 23:40


Mathematics, 22.08.2019 23:40

Health, 22.08.2019 23:40




Mathematics, 22.08.2019 23:40

Social Studies, 22.08.2019 23:40

Mathematics, 22.08.2019 23:50



