
Chemistry, 19.04.2020 03:05 fionademoss6810
Please Help! Let P and V represent the pressure and volume of Xe(g). If a piston is used to reduce the volume of the gas to V/2 at a constant temperature, what is the new pressure in the container in terms of the original pressure, P?
What happens to the average speed of the Xe (g) atoms as the original volume is reduced to V/2 at a constant temperature? Explain.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
Please Help! Let P and V represent the pressure and volume of Xe(g). If a piston is used to reduce t...
Questions







English, 21.02.2020 20:39

Mathematics, 21.02.2020 20:39



History, 21.02.2020 20:39




History, 21.02.2020 20:39





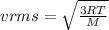
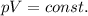
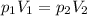
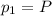 is the initial pressure
is the initial pressure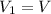 is the initial volume
is the initial volume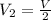 is the final volume
is the final volume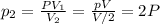
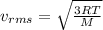
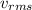 is known as rms speed of the particles in the gas
is known as rms speed of the particles in the gas

