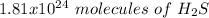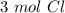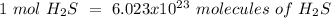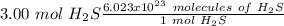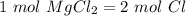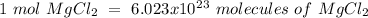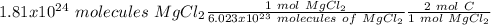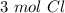Chemistry, 14.11.2019 10:31 MadisonUpky9652
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express your answer numerically in molecules.. calculate the number of moles of cl atoms in 1.81×10^24 formula units of magnesium chloride, mgcl2 .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3
You know the right answer?
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express...
Questions






Health, 24.10.2020 02:30

History, 24.10.2020 02:30


Physics, 24.10.2020 02:30

History, 24.10.2020 02:30


Mathematics, 24.10.2020 02:30

Mathematics, 24.10.2020 02:30


Spanish, 24.10.2020 02:30


Biology, 24.10.2020 02:30