
Chemistry, 20.04.2020 03:25 amakayla57
At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. What is the equilibrium concentration of Mg²⁺ based on the reaction:
Mg(OH)₂ (s) ⇌ Mg²⁺ (aq) + 2 OH⁻ (aq) Kc = 1.80 x 10⁻¹¹

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Asample of rose gold is: 12.0 g gold, 5.0g silver, and 7.0 g copper. what is the percent copper in the sample?
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. What is th...
Questions

World Languages, 08.11.2019 01:31


Mathematics, 08.11.2019 01:31





Mathematics, 08.11.2019 02:31

Biology, 08.11.2019 02:31


History, 08.11.2019 02:31

Mathematics, 08.11.2019 02:31

Mathematics, 08.11.2019 02:31




Biology, 08.11.2019 02:31



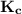 relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction.
relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction. ![\mathbf{K_c = \dfrac{[Mg^{2+}_{(aq)}] [OH^-]^2}{[Mg(OH)_2_{(s)}]} }](/tpl/images/0611/5877/2058a.png)
![\mathbf{1.8 \times 10^{-11} = \dfrac{[Mg^{2+}_{(aq)}] (0.170)^2}{1} }](/tpl/images/0611/5877/d71f0.png)
![\mathbf{ [Mg^{2+}_{(aq)}] = \dfrac{ 1 .8 \times 10^{-11} }{(0.170)^2} }](/tpl/images/0611/5877/a67b7.png)
![\mathbf{ [Mg^{2+}_{(aq)}] =6.22 \times 10^{-10}}](/tpl/images/0611/5877/88f0d.png)
![\mathbf{ [Mg^{2+}_{(aq)}] =0.622 \times 10^{-9}}](/tpl/images/0611/5877/452fc.png)


