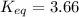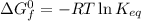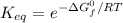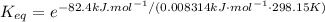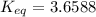Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g)...

Chemistry, 20.04.2020 03:26 officialkk
Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g) + O2(g) + Br2()
given that the standard free energy of formation (AG°f) of NOBr(g) is 82.4 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Questions

History, 21.08.2019 09:30



Mathematics, 21.08.2019 09:30


Social Studies, 21.08.2019 09:30

English, 21.08.2019 09:30

Mathematics, 21.08.2019 09:30

Chemistry, 21.08.2019 09:30

History, 21.08.2019 09:30




Mathematics, 21.08.2019 09:30





Mathematics, 21.08.2019 09:30