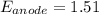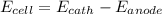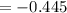Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 15:00
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3
You know the right answer?
The reaction below is spontaneous under standard conditions - true or false? br2(l) + mn2+(aq) → mno...
Questions

Mathematics, 26.01.2021 20:00

Advanced Placement (AP), 26.01.2021 20:00




Mathematics, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00



Mathematics, 26.01.2021 20:00

English, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00


Mathematics, 26.01.2021 20:00




Health, 26.01.2021 20:00

Biology, 26.01.2021 20:00

Mathematics, 26.01.2021 20:00


 [This because it will attract the negative
[This because it will attract the negative