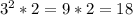Chemistry, 20.04.2020 19:53 angiezavala61
Please consider the following gas phase reaction and its experimentally determined rate law expression. If the concentration of A is tripled and the concentration of B is doubled, the reaction rate would be increased by a factor of:.
A + B → C rate = k[A]^2 [B]
A) 6
B) 9
C) 12
D) 18
E) 36

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2


Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1
You know the right answer?
Please consider the following gas phase reaction and its experimentally determined rate law expressi...
Questions


Mathematics, 24.11.2019 16:31



Mathematics, 24.11.2019 16:31


Social Studies, 24.11.2019 16:31


Geography, 24.11.2019 16:31

History, 24.11.2019 16:31

History, 24.11.2019 16:31

English, 24.11.2019 16:31

History, 24.11.2019 16:31


Mathematics, 24.11.2019 16:31

History, 24.11.2019 16:31

Social Studies, 24.11.2019 16:31


Biology, 24.11.2019 16:31

![r=k[A]^2[B]](/tpl/images/0612/4716/2d753.png)
![r=k[3*A]^2[2*B]](/tpl/images/0612/4716/87b43.png)