

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
At what temperature will a balloon have a volume of 6.08 L if the temperature is 41.0 ℃ when its vol...
Questions

Health, 02.10.2019 18:30


English, 02.10.2019 18:30

English, 02.10.2019 18:30





History, 02.10.2019 18:30

Biology, 02.10.2019 18:30

Mathematics, 02.10.2019 18:30

History, 02.10.2019 18:30




Mathematics, 02.10.2019 18:30




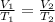

 :
:


