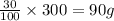Chemistry, 20.04.2020 20:47 Alienhead6187
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution containing 70 g of the compound in 300 mL of water at the same temperature is:
A. unsaturated.
B. saturated.
C. a suspension.
D. supersaturated.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution conta...
Questions

Geography, 25.06.2020 09:01


Mathematics, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01



Mathematics, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01


Mathematics, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01

Health, 25.06.2020 09:01


History, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01


English, 25.06.2020 09:01


Mathematics, 25.06.2020 09:01

Mathematics, 25.06.2020 09:01