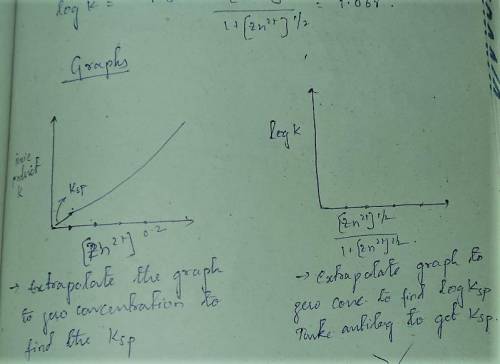
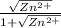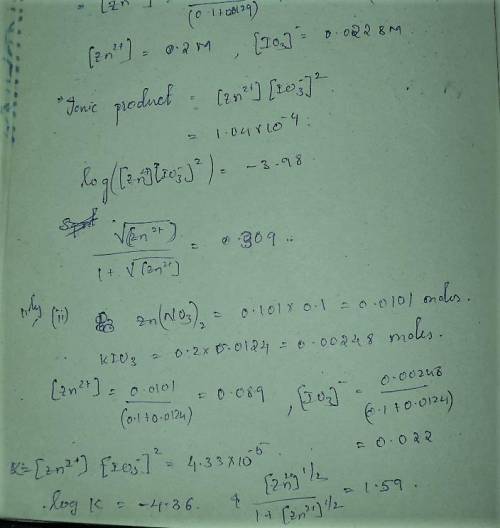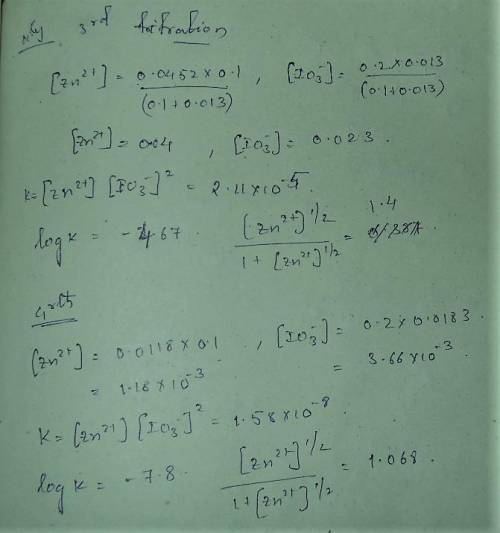A student followed the procedure of this experiment to determine the solubility product of zinc(II) iodate, Zn(IO3)2. Solutions of ZN(NO3)2 of known initial concentrations were titrated with 0.200 M KIO3 solutions to the first appearance of a white precipitate. The following data were collected.
Complete the table below and determine the solubility product constant.
[Zn(NO3)2]0, M Initial 0.226, 0.101 0.0452, 0.0118
[KIO3]0, M Titrant 0.200, 0.200, 0.200, 0.200
V0, mL of Zn(NO3)2 100.0, 100.0, 100.0, 100.0
V, mL of KIO3 titrant 12.9, 12.4, 13.0, 18.3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
A student followed the procedure of this experiment to determine the solubility product of zinc(II)...
Questions