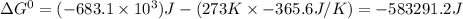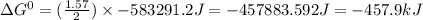For the reaction 2NH3(g) + 2O2(g)N2O(g) + 3H2O(l) H° = -683.1 kJ and S° = -365.6 J/K The standard free energy change for the reaction of 1.57 moles of NH3(g) at 273 K, 1 atm would be kJ. This reaction is (reactant, product) favored under standard conditions at 273 K. Assume that H° and S° are independent of temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2



Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
For the reaction 2NH3(g) + 2O2(g)N2O(g) + 3H2O(l) H° = -683.1 kJ and S° = -365.6 J/K The standard fr...
Questions








Geography, 31.07.2020 01:01


Mathematics, 31.07.2020 01:01

Biology, 31.07.2020 01:01


Social Studies, 31.07.2020 01:01





English, 31.07.2020 01:01


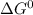 = -457.9 kJ and reaction is product favored.
= -457.9 kJ and reaction is product favored.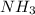
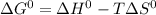 , where T represents temperature in kelvin scale
, where T represents temperature in kelvin scale