Chemistry, 21.04.2020 00:24 vandonquisenberry
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 215. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -155. kJ of work is done on the mixture during the reaction. Calculate the change in energy of the gas mixture during the reaction. Round your answer to 2 significant digits. Is the reaction exothermic or endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3
You know the right answer?
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 215. kJ during a...
Questions



History, 02.12.2020 17:30


Spanish, 02.12.2020 17:30

English, 02.12.2020 17:30



Mathematics, 02.12.2020 17:30


Chemistry, 02.12.2020 17:30

History, 02.12.2020 17:30

Mathematics, 02.12.2020 17:30




English, 02.12.2020 17:30

Mathematics, 02.12.2020 17:30

Mathematics, 02.12.2020 17:30

Biology, 02.12.2020 17:30

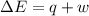
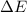 =Change in internal energy
=Change in internal energy