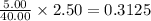A 2.50 L solution contains 5.00 g of NaOH, which has a molecular weight of 40.00
mol
Wha...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Questions

Mathematics, 17.12.2020 22:40

Mathematics, 17.12.2020 22:40

Biology, 17.12.2020 22:40


Mathematics, 17.12.2020 22:40


Mathematics, 17.12.2020 22:40

History, 17.12.2020 22:40


Advanced Placement (AP), 17.12.2020 22:40

History, 17.12.2020 22:40

Mathematics, 17.12.2020 22:40

Mathematics, 17.12.2020 22:40

Computers and Technology, 17.12.2020 22:40


Mathematics, 17.12.2020 22:40

Mathematics, 17.12.2020 22:40

History, 17.12.2020 22:40