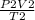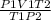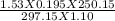Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

You know the right answer?
Find the volume of a balloon of a gas at 842 mm Hg and -23 celcius if it’s volume is 915 milliliters...
Questions

History, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31


Mathematics, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31

Health, 29.10.2019 01:31


History, 29.10.2019 01:31


Mathematics, 29.10.2019 01:31

Mathematics, 29.10.2019 01:31



Computers and Technology, 29.10.2019 01:31





Computers and Technology, 29.10.2019 01:31

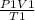 =
=