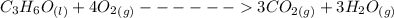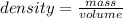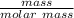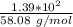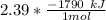Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover. c3h6o (l) + 4 o2 (g) à 3 co2 (g) + 3 h2o (g) ∆horxn = -1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions

Mathematics, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01








Advanced Placement (AP), 20.08.2020 01:01










Mathematics, 20.08.2020 01:01