Chemistry, 21.04.2020 08:19 ninaaforever
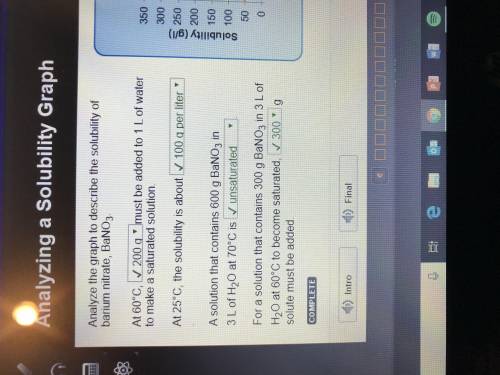
Analyze the graph to describe the solubility of barium nitrate, BaNO3. At 60°C, must be added to 1 L of water to make a saturated solution. At 25°C, the solubility is about . A solution that contains 600 g BaNO3 in 3 L of H2O at 70°C is . For a solution that contains 300 g BaNO3 in 3 L of H2O at 60°C to become saturated, g solute must be added.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Analyze the graph to describe the solubility of barium nitrate, BaNO3. At 60°C, must be added to 1 L...
Questions


Mathematics, 24.03.2021 20:50

Mathematics, 24.03.2021 20:50






History, 24.03.2021 21:00

Chemistry, 24.03.2021 21:00

Business, 24.03.2021 21:00


Mathematics, 24.03.2021 21:00


Chemistry, 24.03.2021 21:00