
Chemistry, 21.04.2020 15:29 hindjssnsk
A chemist must dilute 47.2 mL of 150. mM aqueous sodium nitrate solution until the concentration falls to . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3


Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
A chemist must dilute 47.2 mL of 150. mM aqueous sodium nitrate solution until the concentration fal...
Questions

Physics, 24.11.2021 19:00

Mathematics, 24.11.2021 19:00

Biology, 24.11.2021 19:00


Mathematics, 24.11.2021 19:00


Mathematics, 24.11.2021 19:00





History, 24.11.2021 19:00

Social Studies, 24.11.2021 19:00


Mathematics, 24.11.2021 19:00



Social Studies, 24.11.2021 19:00


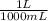 = 0.295 L
= 0.295 L


