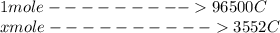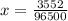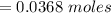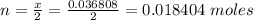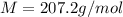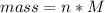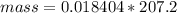Chemistry, 21.04.2020 15:30 sophcent5828
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate is reduced to lead at the cathode and oxidized to solid lead(II) oxide at the anode. Suppose a current of is fed into a car battery for seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentiall...
Questions


Computers and Technology, 04.11.2019 03:31


Spanish, 04.11.2019 03:31

Mathematics, 04.11.2019 03:31


English, 04.11.2019 03:31



History, 04.11.2019 03:31

World Languages, 04.11.2019 03:31


Geography, 04.11.2019 03:31


Biology, 04.11.2019 03:31

Biology, 04.11.2019 03:31




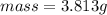
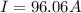
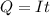
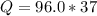
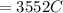
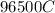
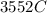 would contain how many moles
would contain how many moles