Chemistry, 21.04.2020 15:24 jasminecoronetti44
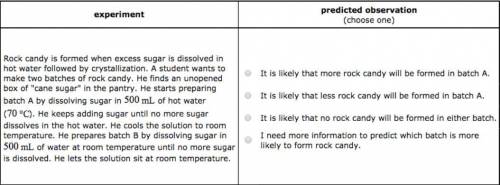
Rock candy is formed when excess sugar is dissolved in hot water followed by crystallization. A student wants to make two batches of rock candy. He finds an unopened box of "cane sugar" in the pantry. He starts preparing batch A by dissolving sugar in of hot water (). He keeps adding sugar until no more sugar dissolves in the hot water. He cools the solution to room temperature. He prepares batch B by dissolving sugar in of water at room temperature until no more sugar is dissolved. He lets the solution sit at room temperature. It is likely that less rock candy will be formed in batch A. It is likely that no rock candy will be formed in either batch. I need more information to predict which batch is more likely to form rock candy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Rock candy is formed when excess sugar is dissolved in hot water followed by crystallization. A stud...
Questions




Chemistry, 03.03.2021 02:30

Social Studies, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30


Mathematics, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30

History, 03.03.2021 02:30

Advanced Placement (AP), 03.03.2021 02:30


Biology, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30

Mathematics, 03.03.2021 02:30