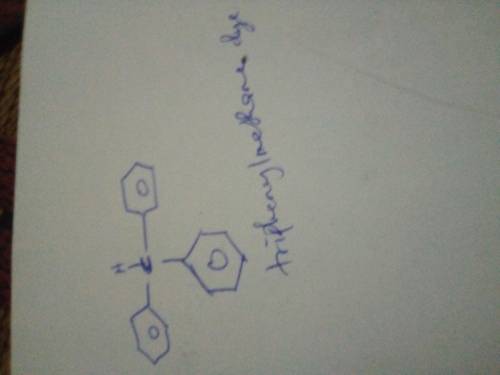
Triphenylmethanol is insoluble in water, but when it is treated with concentrated H2SO4, a bright yellow solution results. As this yellow solution is diluted with water, its color disappears, and a precipitate of triphenylmethanol reappears. Suggest a structure for the bright yellow species.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Triphenylmethanol is insoluble in water, but when it is treated with concentrated H2SO4, a bright ye...
Questions


Spanish, 31.07.2019 03:50



Social Studies, 31.07.2019 03:50

History, 31.07.2019 03:50

Mathematics, 31.07.2019 03:50

Mathematics, 31.07.2019 03:50

Mathematics, 31.07.2019 03:50

Arts, 31.07.2019 03:50

Arts, 31.07.2019 03:50

Arts, 31.07.2019 03:50

Arts, 31.07.2019 03:50

Arts, 31.07.2019 03:50



Mathematics, 31.07.2019 03:50


Spanish, 31.07.2019 03:50

Spanish, 31.07.2019 03:50