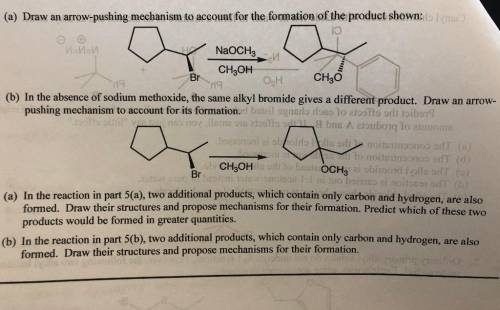
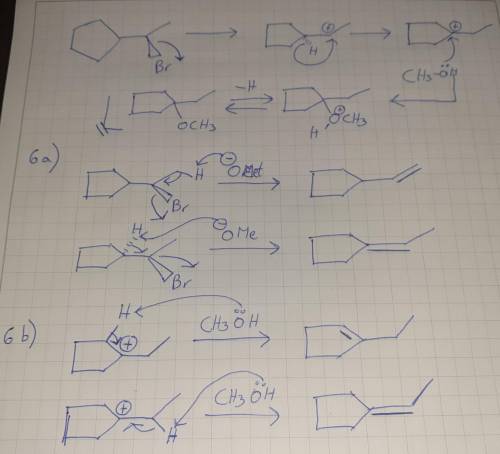
Chemistry, 21.04.2020 16:56 bankrollbaby01
In the absence of sodium methoxide, the same alkyl bromide gives a different product. Draw an arrowpushing mechanism to account for its formation. 6. (a) In the reaction in part 5(a), two additional products, which contain only carbon and hydrogen, are also formed. Draw their structures and propose mechanisms for their formation. Predict which of these two products would be formed in greater quantities. (b) In the reaction in part 5(b), two additional products, which contain only carbon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
In the absence of sodium methoxide, the same alkyl bromide gives a different product. Draw an arrowp...
Questions

Mathematics, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00


Social Studies, 26.06.2021 01:00



Physics, 26.06.2021 01:00

Mathematics, 26.06.2021 01:00


English, 26.06.2021 01:00







Mathematics, 26.06.2021 01:00