
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K the KC is 1.1x10-2. Given that 1.0g of phosphorous pentachloride is added to a 250mL reaction flask, find the percent decomposition after the system has reached equilibrium. PCl_5(g) PCl_3(g) Cl_2(g) K_C

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
For the decomposition of phosphorous pentachloride to phosphorous trichloride and chlorine at 400K t...
Questions

Mathematics, 06.10.2020 14:01

Health, 06.10.2020 14:01


Advanced Placement (AP), 06.10.2020 14:01



English, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01



Biology, 06.10.2020 14:01

Arts, 06.10.2020 14:01


Spanish, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Social Studies, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

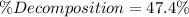
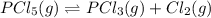
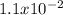 and the initial concentration of phosphorous pentachloride is:
and the initial concentration of phosphorous pentachloride is:![[PCl_5]_0=\frac{1.0gPCl_5*\frac{1molPCl_5}{208.24gPCl_5} }{250mL*\frac{1L}{1000mL} } =0.019M](/tpl/images/0614/9385/f2a4f.png)
![Kc=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0614/9385/c6686.png)
 occurring due to the reaction extent and the concentrations at equilibrium (ICE table methodology):
occurring due to the reaction extent and the concentrations at equilibrium (ICE table methodology):![Kc=\frac{(x)(x)}{[PCl_5]_0-x}=\frac{x^2}{0.019-x}=1.1x10^{-2}](/tpl/images/0614/9385/3162f.png)
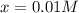
![[PCl_5]_{eq}=[PCl_5]_0-x=0.019M-0.01M\\](/tpl/images/0614/9385/8b1c4.png)
![[PCl_5]_{eq}=0.009M](/tpl/images/0614/9385/82584.png)
![\% Decomposition=\frac{[PCl_5]_0}{[PCl_5]_{eq}}*100\%=\frac{0.009M}{0.019M} *100\%\\\\\% Decomposition=47.4\%](/tpl/images/0614/9385/7bb6c.png)


