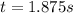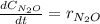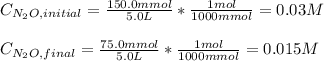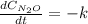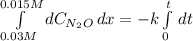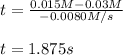Chemistry, 21.04.2020 17:30 amandajbrewerdavis
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of ·0.0080Ms−1: 2N2O(g)→2N2(g)+O2(g) Suppose a 5.0L flask is charged under these conditions with 150.mmol of dinitrogen monoxide. After how much time is there only 75.0mmol left? You may assume no other reaction is important.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate...
Questions

History, 20.10.2020 04:01

Biology, 20.10.2020 04:01

Computers and Technology, 20.10.2020 04:01

Health, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01




Mathematics, 20.10.2020 04:01


Health, 20.10.2020 04:01

History, 20.10.2020 04:01

Spanish, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01

Advanced Placement (AP), 20.10.2020 04:01

Mathematics, 20.10.2020 04:01



Mathematics, 20.10.2020 04:01

English, 20.10.2020 04:01