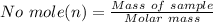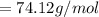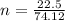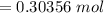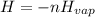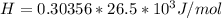Chemistry, 21.04.2020 18:07 roseemariehunter12
The following information is given for ether, C2H5OC2H5, at 1atm: boiling point = 34.6 °C Hvap(34.6 °C) = 26.5 kJ/mol specific heat liquid = 2.32 J/g°C /At a pressure of 1 atm, what is H in kJ for the process of condensing a 22.5 g sample of gaseous ether at its normal boiling point of 34.6 °C.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
The following information is given for ether, C2H5OC2H5, at 1atm: boiling point = 34.6 °C Hvap(34.6...
Questions


English, 12.10.2020 22:01

Business, 12.10.2020 22:01


Biology, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01

English, 12.10.2020 22:01

Physics, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01







Mathematics, 12.10.2020 22:01

Chemistry, 12.10.2020 22:01


English, 12.10.2020 22:01

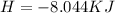
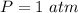
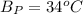
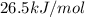

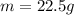
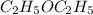 is given as
is given as