Chemistry, 21.04.2020 18:51 tfaulk2884
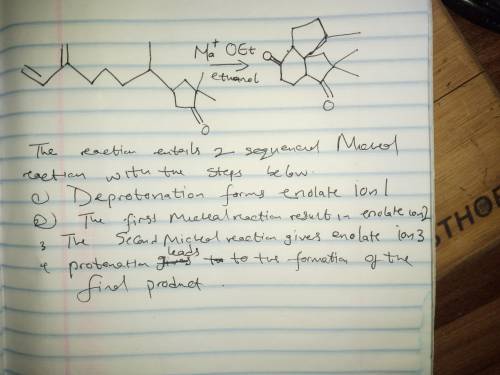
This reaction involves two successive Michael reactions, and has the following steps: 1. Deprotonation forms enolate ion 1; 2. The first Michael reaction forms enolate ion 2; 3. The second Michael reaction forms enolate ion 3; 4. Protonation leads to the final product. Write the mechanism out on a sheet of paper, and then draw the structure of enolate ion 1.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
This reaction involves two successive Michael reactions, and has the following steps: 1. Deprotonati...
Questions

Physics, 05.07.2019 06:00

Mathematics, 05.07.2019 06:00








Mathematics, 05.07.2019 06:00

Social Studies, 05.07.2019 06:00


Mathematics, 05.07.2019 06:00

Computers and Technology, 05.07.2019 06:00

Social Studies, 05.07.2019 06:00

History, 05.07.2019 06:00

Mathematics, 05.07.2019 06:00

Mathematics, 05.07.2019 06:00

Mathematics, 05.07.2019 06:00

Biology, 05.07.2019 06:00