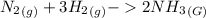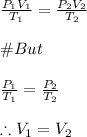Chemistry, 21.04.2020 20:17 amypeace1978
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L. The following reaction takes place: N2(g) 3 H2(g) 2 NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant. g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1


You know the right answer?
A sample of gas contains 0.1100 mol of N2(g) and 0.3300 mol of H2(g) and occupies a volume of 20.5 L...
Questions




History, 01.07.2019 18:00

Physics, 01.07.2019 18:00


Social Studies, 01.07.2019 18:00

Mathematics, 01.07.2019 18:00


Arts, 01.07.2019 18:00



Mathematics, 01.07.2019 18:00


Computers and Technology, 01.07.2019 18:00


History, 01.07.2019 18:00

Mathematics, 01.07.2019 18:00