Chemistry, 21.04.2020 20:19 siriuskitwilson9408
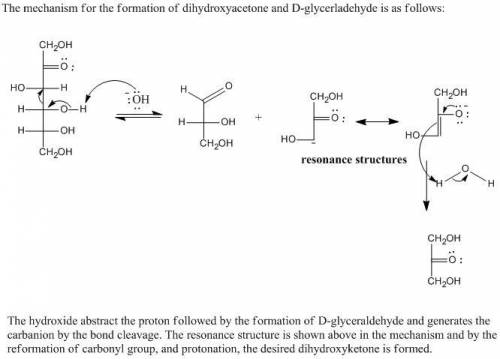
On treatment with aqueous base, D-fructose slowly undergoes cleavage to form dihydroxyacetone and D-glyceraldehyde (in low yield). The second step of this reaction produces two organic products, one of which is an anion with two resonance structures. Draw the structures of the two products formed in the second step of the reaction. Draw only one resonance structure for the anionic product.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
On treatment with aqueous base, D-fructose slowly undergoes cleavage to form dihydroxyacetone and D-...
Questions

Computers and Technology, 30.01.2021 01:00





English, 30.01.2021 01:00


Business, 30.01.2021 01:00



Spanish, 30.01.2021 01:00

Chemistry, 30.01.2021 01:00


Geography, 30.01.2021 01:00


Chemistry, 30.01.2021 01:00


English, 30.01.2021 01:00

Geography, 30.01.2021 01:00