
Chemistry, 21.04.2020 20:48 25jzaldivar
The decomposition reaction 2 NOCl → 2 NO + Cl_2 has a rate law that is second order with respect to [NOCl], where k = 3.2 M^{-1}s^{-1} at a certain temperature. If the initial concentration of NOCl is 0.076 M, how many seconds will it take for [NOCl] to decrease to 0.042 M at this temperature? Do not enter units with your numerical answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
The decomposition reaction 2 NOCl → 2 NO + Cl_2 has a rate law that is second order with respect to...
Questions

Mathematics, 19.09.2019 22:30



SAT, 19.09.2019 22:30

English, 19.09.2019 22:30





Mathematics, 19.09.2019 22:30




Mathematics, 19.09.2019 22:30

Mathematics, 19.09.2019 22:30


Mathematics, 19.09.2019 22:30

History, 19.09.2019 22:30


History, 19.09.2019 22:30

![\frac{1}{[NOCl]}=kt+\frac{1}{[NOCl]_{0}}](/tpl/images/0615/7124/81c05.png)
![[NOCl]_{0}](/tpl/images/0615/7124/069d3.png) is initial concentration of NOCl and k is rate constant.
is initial concentration of NOCl and k is rate constant.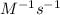 and [NOCl] = 0.042 M
and [NOCl] = 0.042 M![\frac{1}{0.042M}=[3.2M^{-1}s^{-1}\times t]+\frac{1}{0.076M}](/tpl/images/0615/7124/ee865.png)


