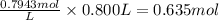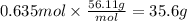Chemistry, 21.04.2020 20:47 romeroalexis817
A chemist must prepare 800mL of potassium hydroxide solution with a pH of 13.90 at 25C.?
He will do this in three steps.
1. fill a 800mL volumetric flask about halfway with distilled water.
2. weigh out a small amount of solid potassium hydroxide and add it to the flask.
3. fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
A chemist must prepare 800mL of potassium hydroxide solution with a pH of 13.90 at 25C.?
He wi...
He wi...
Questions




Mathematics, 23.01.2020 19:31

History, 23.01.2020 19:31

Mathematics, 23.01.2020 19:31

Spanish, 23.01.2020 19:31

Mathematics, 23.01.2020 19:31

Social Studies, 23.01.2020 19:31



Chemistry, 23.01.2020 19:31



Mathematics, 23.01.2020 19:31


Biology, 23.01.2020 19:31