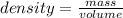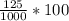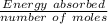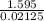Chemistry, 21.04.2020 22:43 yoyoho6218
You mix 125 mL of 0.170 M with 50.0 mL of 0.425 M in a coffee-cup calorimeter, and the temperature of both solutions rises from 20.20 °C before mixing to 22.17 °C after the reaction. What is the enthalpy of reaction per mole of ? Assume the densities of the solutions are all 1.00 g/mL, and the specific heat capacities of the solutions are 4.2 J/g · K. Enthalpy of reaction = kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
You mix 125 mL of 0.170 M with 50.0 mL of 0.425 M in a coffee-cup calorimeter, and the temperature o...
Questions

English, 12.04.2021 04:10



Mathematics, 12.04.2021 04:10

Spanish, 12.04.2021 04:10

Mathematics, 12.04.2021 04:10



Physics, 12.04.2021 04:10

Chemistry, 12.04.2021 04:10


Mathematics, 12.04.2021 04:10


English, 12.04.2021 04:10


Mathematics, 12.04.2021 04:10

Spanish, 12.04.2021 04:10

History, 12.04.2021 04:10


Biology, 12.04.2021 04:10