
Chemistry, 21.04.2020 22:41 mildredelizam
In acidic solution, the sulfate ion can be used to react with a number of metal ions. One such reaction is SO42−(aq)+Sn2+(aq)→H2SO3(aq)+Sn4+(a q) Since this reaction takes place in acidic solution, H2O(l) and H+(aq) will be involved in the reaction. Places for these species are indicated by the blanks in the following restatement of the equation: SO42−(aq)+Sn2+(aq)+ –––→H2SO3(aq)+Sn4+(aq)+ ––– Part B What are the coefficients of the reactants and products in the balanced equation above? Remember to include H2O(l) and OH−(aq) in the blanks where appropriate. Your answer should have six terms. Enter the equation coefficients in order separated by commas (e. g., 2,2,1,4,4,3). Include coefficients of 1, as required, for grading purposes.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2


Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
In acidic solution, the sulfate ion can be used to react with a number of metal ions. One such react...
Questions


Mathematics, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

English, 12.12.2020 16:00




History, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

English, 12.12.2020 16:00

English, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00



Chemistry, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00

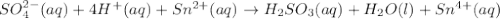
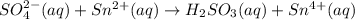

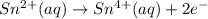 ....[1]
....[1]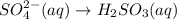
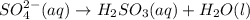
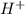 on the required side:
on the required side: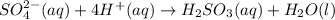
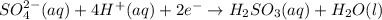 ..[2]
..[2]

