Chemistry, 21.04.2020 22:04 Irenesmarie8493
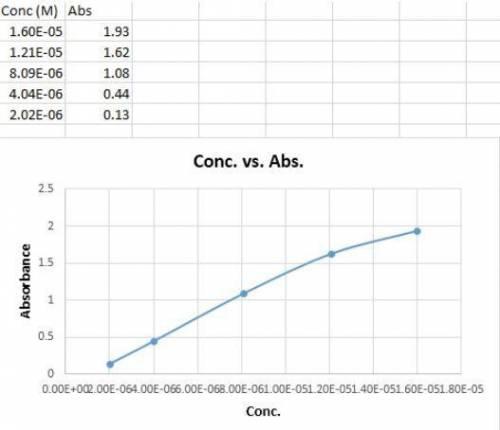
You make dilutions of curcumin stock solution and measure the absorbance of each dilution to obtain the following data: Conc. (M) Abs. 1.60E-05 1.93 1.21E-05 1.62 8.09E-06 1.08 4.04E-06 0.44 2.02E-06 0.13 Which data points should you keep when making your calibration curve? Choose all that apply. (Hint: Plot the data.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
You know the right answer?
You make dilutions of curcumin stock solution and measure the absorbance of each dilution to obtain...
Questions

Arts, 04.09.2021 19:20

Biology, 04.09.2021 19:20










Mathematics, 04.09.2021 19:20


Mathematics, 04.09.2021 19:20

Engineering, 04.09.2021 19:20


Mathematics, 04.09.2021 19:20

Physics, 04.09.2021 19:20

Mathematics, 04.09.2021 19:20