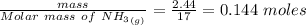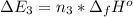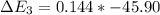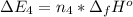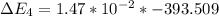Chemistry, 21.04.2020 22:23 asdf334asdf334
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 296.84 kJ/mol) Enthalpy change = kJ 0.22 mol of decomposes to and ( for = –90.83 kJ/mol) Enthalpy change = kJ 2.44 g of is formed from and excess ( for = –45.90 kJ/mol) Enthalpy change = kJ mol of carbon is oxidized to ( for = –393.509 kJ/mol) Enthalpy change = kJ

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1


Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 2...
Questions



English, 11.03.2021 01:00




Mathematics, 11.03.2021 01:00

Biology, 11.03.2021 01:00




Mathematics, 11.03.2021 01:00






Mathematics, 11.03.2021 01:00


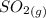 (
( 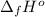 for
for 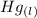 and
and 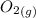 (
( 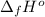 for
for 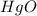 = –90.83
= –90.83 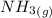 is formed from and excess
is formed from and excess 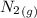 and excess
and excess 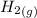 (
( 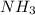 = –45.90 kJ/mol)
= –45.90 kJ/mol) 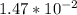 mol of carbon is oxidized to
mol of carbon is oxidized to 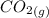 (
( 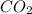 = –393.509 kJ/mol)
= –393.509 kJ/mol) 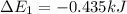
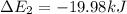
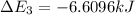
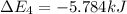
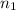 ) =
) = 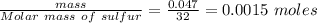
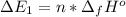
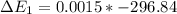
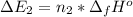
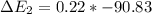
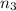 ) =
) =