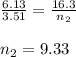Chemistry, 22.04.2020 00:10 Affousietta
A flexible container at an initial volume of 6.13 L contains 3.51 mol of gas. More gas is then added to the container until it reaches a final volume of 16.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container. number of moles of gas: mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
A flexible container at an initial volume of 6.13 L contains 3.51 mol of gas. More gas is then added...
Questions


Biology, 28.01.2020 14:44


History, 28.01.2020 14:44

Mathematics, 28.01.2020 14:44



Biology, 28.01.2020 14:44

Spanish, 28.01.2020 14:44

History, 28.01.2020 14:44

Social Studies, 28.01.2020 14:44

History, 28.01.2020 14:44



Mathematics, 28.01.2020 14:44


Mathematics, 28.01.2020 14:44



English, 28.01.2020 14:44

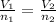
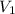 and
and 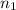 are the initial volume and number of moles
are the initial volume and number of moles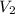 and
and 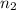 are the final volume and number of moles
are the final volume and number of moles