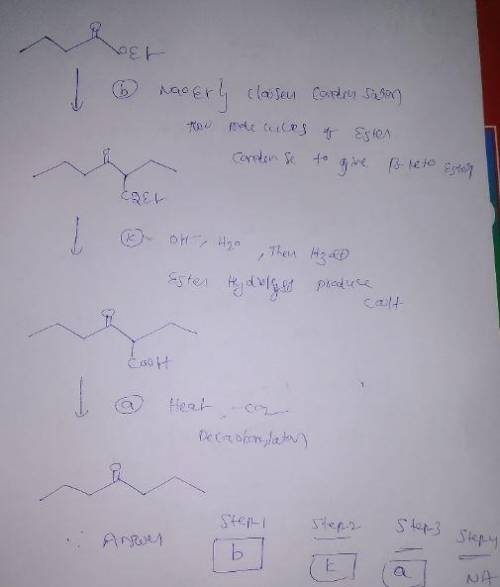
Problem 19.24a Using the reagents below, list in order (by letter, no period) those necessary to convert the starting material into the given product: Note: More than 1 mole of the starting material may be used. Not all spaces provided may be needed. Type "na" in any space where you have no reagent. a. heat, -CO2 b. NaOEt c. (CH3CH2)2CuLi d. CH2Cl2, PCC e. C3H7C(O)CH(C2H5)C(O)C2H5 f. CH3CH2Li g. CH3C(O)Cl, AlCl3 h. NBS, ROOR i. H2NC(O)NH2 j. HN(CH3)2 k. OH-, H2O, heat then H3O l. H3O

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
Problem 19.24a Using the reagents below, list in order (by letter, no period) those necessary to con...
Questions





SAT, 28.12.2021 23:10

SAT, 28.12.2021 23:10



Physics, 28.12.2021 23:10



Health, 28.12.2021 23:10

Biology, 28.12.2021 23:10

History, 28.12.2021 23:10


SAT, 28.12.2021 23:10