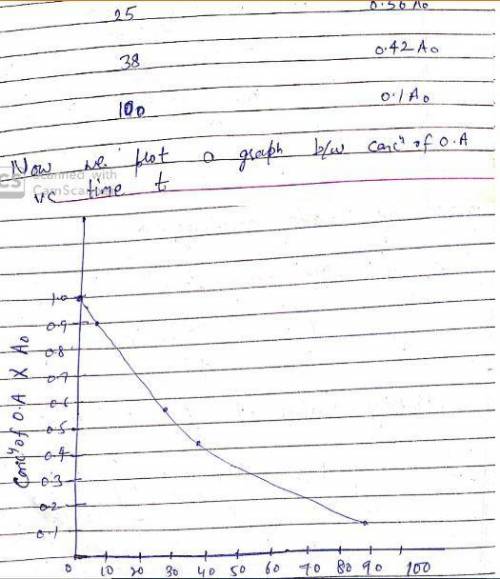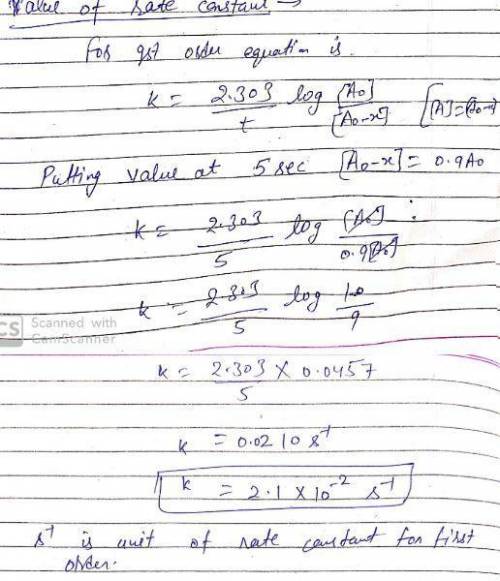The decarboxylation of oxaloacetate into pyruvate and CO2 at pH 5 and 25C is 10% complete in 5 s, 44% complete in 25 s, 58% complete in 38 s, and 90% complete in 100 s. What is the reaction order with respect to oxalacetate and the rate constant (including units) for the reaction? Show your work.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

You know the right answer?
The decarboxylation of oxaloacetate into pyruvate and CO2 at pH 5 and 25C is 10% complete in 5 s, 44...
Questions

History, 18.12.2022 14:00

Social Studies, 18.12.2022 16:40

Social Studies, 18.12.2022 18:40

Social Studies, 18.12.2022 20:00




Social Studies, 18.12.2022 22:30




Social Studies, 19.12.2022 01:00

Computers and Technology, 19.12.2022 01:00





Social Studies, 19.12.2022 03:10


Mathematics, 19.12.2022 03:50