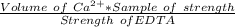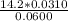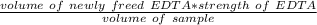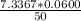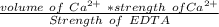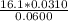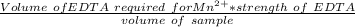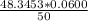Chemistry, 22.04.2020 01:05 cheeraly09
A 50.0 mL sample containing Cd2+ and Mn2+ was treated with 64.0 mL of 0.0600 M EDTA . Titration of the excess unreacted EDTA required 16.1 mL of 0.0310 M Ca2+ . The Cd2+ was displaced from EDTA by the addition of an excess of CN− . Titration of the newly freed EDTA required 14.2 mL of 0.0310 M Ca2+ . What are the concentrations of Cd2+ and Mn2+ in the original solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

You know the right answer?
A 50.0 mL sample containing Cd2+ and Mn2+ was treated with 64.0 mL of 0.0600 M EDTA . Titration of t...
Questions


Health, 10.12.2020 18:10

History, 10.12.2020 18:10



History, 10.12.2020 18:10







Mathematics, 10.12.2020 18:10

Mathematics, 10.12.2020 18:10

Mathematics, 10.12.2020 18:10


English, 10.12.2020 18:10

Chemistry, 10.12.2020 18:10

Mathematics, 10.12.2020 18:10

World Languages, 10.12.2020 18:10

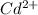 in the original solution= 0.0088 M
in the original solution= 0.0088 M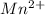 in the original solution = 0.058 M
in the original solution = 0.058 M