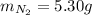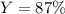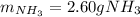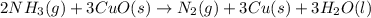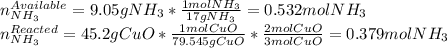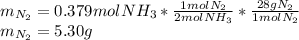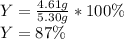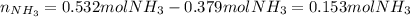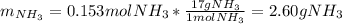Chemistry, 22.04.2020 01:43 sayedabdullah
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3 reacted with 45.2 g of CuO?(5 points) b. How many grams of N2 can be made?(10 points) c. If 4.61 g of N2 are made, what is the percent yield? (5 points) d. What is the mass of the excess reactant that remains after the reaction. (10 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
2 NH3(g) + 3 CuO(s) → N2(g) + 3 Cu(s) + 3 H2O(l) a. What is the limiting reagent when 9.05 g of NH3...
Questions





History, 08.06.2020 02:57



Mathematics, 08.06.2020 02:57





History, 08.06.2020 02:57

Mathematics, 08.06.2020 02:57

Mathematics, 08.06.2020 02:57


Mathematics, 08.06.2020 02:57

Mathematics, 08.06.2020 02:57