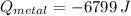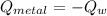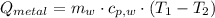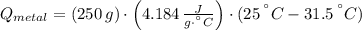Chemistry, 22.04.2020 02:39 rodolfoperezzz1332
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initially at 25.0 oC. The final temperature of the water is 31.5 oC. What is the heat change of the metal in joules? The specific heat of water is 4.184 J/goC

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3

You know the right answer?
A 20.0 g piece of a metal is heated and place into a calorimeter containing 250.0 g of water initial...
Questions

History, 25.01.2021 19:00

Social Studies, 25.01.2021 19:00

Mathematics, 25.01.2021 19:00


Mathematics, 25.01.2021 19:00


Mathematics, 25.01.2021 19:00

Mathematics, 25.01.2021 19:00



Chemistry, 25.01.2021 19:00




Mathematics, 25.01.2021 19:00


Mathematics, 25.01.2021 19:00


Mathematics, 25.01.2021 19:00

Mathematics, 25.01.2021 19:00