
Chemistry, 22.04.2020 01:56 julielebo8
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction system. b. the increased molecular disorder that occurs in the system. c. the energy given off to the surroundings. d. the direction in which a net reaction occurs. e. the excess entropy given off to the surroundings

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
The free-energy change of a reaction is a measure of a. the excess entropy given off to the reaction...
Questions

Physics, 12.09.2021 05:00

History, 12.09.2021 05:00




Chemistry, 12.09.2021 05:10


Mathematics, 12.09.2021 05:10




Mathematics, 12.09.2021 05:10

Biology, 12.09.2021 05:10

English, 12.09.2021 05:10


Mathematics, 12.09.2021 05:10

Physics, 12.09.2021 05:10


Mathematics, 12.09.2021 05:10

Health, 12.09.2021 05:10

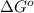 . Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.
. Free-energy helps in determining the direction of a chemical reaction like if it is taking place in forward or backward reaction.

