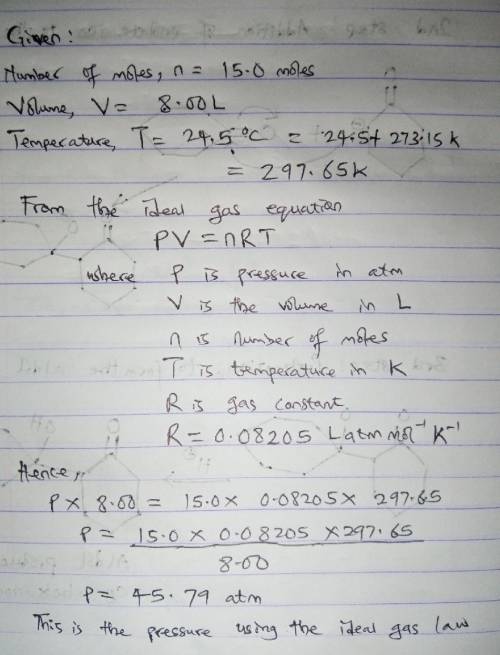Chemistry, 22.04.2020 02:08 awesomegrill
15.0 moles of gas are in a 8.00 LL tank at 24.5 ∘C∘C . Calculate the difference in pressure between methane and an ideal gas under these conditions. The van der Waals constants for methane are a=2.300L2⋅atm/mol2a=2.300L2⋅atm/mol 2 and b=0.0430 L/molb=0.0430 L/mol .

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1

Chemistry, 23.06.2019 15:20
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2
You know the right answer?
15.0 moles of gas are in a 8.00 LL tank at 24.5 ∘C∘C . Calculate the difference in pressure between...
Questions

Social Studies, 18.10.2020 15:01


Chemistry, 18.10.2020 15:01




Physics, 18.10.2020 15:01

French, 18.10.2020 15:01

Social Studies, 18.10.2020 15:01

Social Studies, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01



Biology, 18.10.2020 15:01



History, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Biology, 18.10.2020 15:01

Physics, 18.10.2020 15:01