
Chemistry, 22.04.2020 02:12 trinati6965
Calculate the DH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide. DH°f means delta or change of heat of formation DH°f [CaCO3(s)] = –1206.9 kJ/mol; DH°f [CaO(s)] = –635.1 kJ/mol; DH°f [CO2(g)] = –393.5 kJ/mol CaCO3(s) --> CaO(s) + CO2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

You know the right answer?
Calculate the DH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide....
Questions


Mathematics, 27.02.2021 06:20

Mathematics, 27.02.2021 06:20




Mathematics, 27.02.2021 06:20

English, 27.02.2021 06:30


Biology, 27.02.2021 06:30

Social Studies, 27.02.2021 06:30







Mathematics, 27.02.2021 06:30

Mathematics, 27.02.2021 06:30

Social Studies, 27.02.2021 06:30

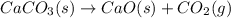
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(CaO(s))})+(1\times \Delta H^0f_{CO_2}]-[(1\times \Delta H^o_f_{(CaCO_3(s))})]](/tpl/images/0617/0361/6353c.png)
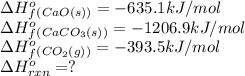
![\Delta H^o_{rxn}=[(1\times (-635.1))+(1\times (-393.5))]-[(1\times (-1206.9))]](/tpl/images/0617/0361/be80a.png)


