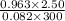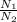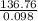You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 [infinity]C. The He is used to fill balloons to a volume of 2.50 L at 732 mm Hg and 27 [infinity]C. How many balloons can be filled with He? Assume that the cylinder can provide He until its internal pressure reaches 1.00 atm (i. e., there are 131 atmospheres of usable He in the cylinder).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Select the correct answer from each drop-down menu. daniel and sanya are scientists. daniel is studying whether the increasing frequency of tropical storms is affecting coastal erosion. sanya is investigating whether the discharge from industrial plants has any impact on the ph concentration of freshwater swamps in the surrounding area. which fields of science are daniel’s and sanya’s studies most closely related to? daniel’s field of study is related to science, and sanya’s field of study is related to .
Answers: 3

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 [infinity]C. T...
Questions

Mathematics, 30.05.2020 00:03

Mathematics, 30.05.2020 00:03

Mathematics, 30.05.2020 00:03

Social Studies, 30.05.2020 00:03


Mathematics, 30.05.2020 00:03

Physics, 30.05.2020 00:03

History, 30.05.2020 00:03


Mathematics, 30.05.2020 00:03


History, 30.05.2020 00:03



Mathematics, 30.05.2020 00:03

Chemistry, 30.05.2020 00:03



Physics, 30.05.2020 00:03

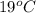 = (19 + 273) K
= (19 + 273) K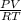
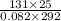
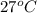 = (27 + 273) K
= (27 + 273) K